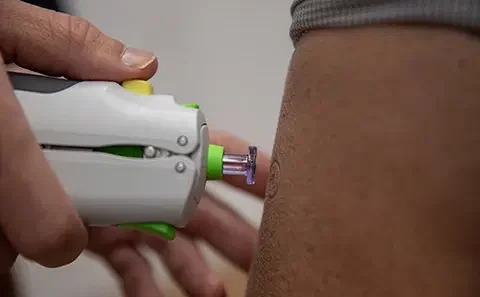
A study conducted on the Intradermal, ID vaccine administration using the Tropis ID Needle-free Injection System, NFIS in Nigeria has shown that switching to needle-free delivered fIPV could save the Nigeria immunisation program $49.51 million over a 5-year period Needle-Free Immunisation.
The 6 months study also indicated that among those vaccinated with Tropis, IPV2 coverage was 11.2% higher, compared with needle and syringe (SoC).
Other results shown in the study surveyed on 97,000 households in the two sample states affirmed that the Needle-free method of immunisation called Tropis is an effective intervention for increasing coverage of immunisation, IPV2.
On a relative basis, the study shows that odds of receiving 2 doses of IPV are doubled when Tropis is used, adding that all intervention scenarios demonstrate cost savings compared to SoC.
According to the study, there was an incremental cost savings with needle-free ranged from $0.07 to $1.00, up to 47% total immunization cost savings compared to SoC.
Presenting the project context, the team Lead, Mr. Labarre Paul said caregivers, Health Care Workers and stakeholders found needle-free: highly acceptable, with the needle-free aspect highly valued and preferred over SoC. And for caregivers, he said the preference was: 95%, 89% easier; 78% safer.
And when Compared with SoC, Paul said needle-free did not require additional time for administration but actually saves a few seconds per administration.
Overall, he said that the study results were positive across the board indicating that Tropis is effective at increasing coverage, decreasing program costs, and is scalable for routine immunisation use.
Recall that in September 2022, Pharmajet, Jhpiego, PATH, Sydani Group and the Nigeria National Primary Health Care Development Agency, NPHCDA received a multi-year grant from the US agency for International Development, USAID to evaluate the impact of intradermal , ID vaccine administration using the Tropis ID Needle-free Injection System, NFIS in Nigeria.
The study measured the effectiveness of coverage and costs of using Tropis ID for fractional inactivated poliovirus vaccine (fIPV) delivery compared with standard of care intramuscular delivery using needle and syringe.
The mixed methods study also assessed the acceptability, feasibility, sustainability and scalability of the introduction of Tropis IPV delivery into the Nigerian Expanded Immunization (EPI) program.
From October 2022 to July 2024 (inclusive of the 6-month implementation period), the team conducted an implementation research study in Kano and Oyo states.
The study aimed to test the comparative effectiveness of Tropis for fIPV in routine immunization services as compared to standard-of-care (Soc) vaccination practice (0.5 ml IM injection with traditional needle and syringe) in improving 2-dose IPV coverage (IPV2) among children aged less than one year; to assess the total immunization cost impact of using Tropis for fIPV delivery as compared to SoC vaccination practice and to understand the acceptability, feasibility, scalability, and sustainability of fIPV Tropis delivery.
In his remarks, the Director Health Population and Nutrition Office at USAID/Nigeria, Sinu Kurian, said the collaborative work and its implementation in two states of Kano and Oyo in Nigeria, marked a significant step towards ensuring equitable and sustainable polio immunization coverage for Nigerian children.
He affirmed that the findings will not only help guide decisions in Nigeria but will also contribute to global discussions on the future of polio eradication efforts.
''This intervention has allowed us to explore innovative strategies in vaccine delivery whilst addressing the persistent challenges of cost and vaccine supply shortages - issues that limit the timely introduction and scaling up of Inactivated Polio Vaccine (IPV).
The challenges, though global, takes on the unique complexities in countries like Nigeria, where strong evidence is needed to support the transition to fractional IPV doses as recommended by the World Health Organization.
"Through USAID's investment in this study, we sought to generate in-country evidence on the acceptability, feasibility, and cost-effectiveness of deploying the Tropis device to deliver fractional doses of IPV. The evidence provided at this meeting is essential for Nigeria's readiness to embrace this innovation at a national scale.''
He however, assured that USAID remains committed to supporting Nigeria in achieving its health goals, ensuring every child receives lifesaving vaccinations and interventions.
In his own remarks, the Senior Special Adviser to the minister of health, the Ministry of health, Dr. Emmanuel Oduh , said the study aligned with the 3rd health care agenda of the minister which aims to unlock the healthcare value chain, leading to local manufacturing of medical equipment,l help in policy formulation, and make health manufacturers keying into in-country manufacturing of healthcare services and devices.
''This study comes with a new strategy for administration of vaccines and is a strategy been implemented through the instrumentality of evidence , research. It is the first time a strategy is being implemented through a research based on evidence generated locally. The benefits are obvious. It improves coverage of targeted population, and also cost savings and feasibility, both health care workers and patients find it convenience. That conventional response that comes with use of needles or vaccines is not there. We need to receive this report reviewed and see how it can influence policies.''
The Director, Disease Control and Immunization at National Primary Healthcare Development Agency, Dr. Garuba Ahmed Rufai said ''innovations are always welcome. With this study, we can be able to provide vaccination without having children crying. And one of the reasons parents withdraw children from immunization is because of the way children cry with vaccine. This is a good one for us.
In terms of how the country is going to accept it, he said, ''we will look at all the parameters in terms of what it will benefit us, cost savings, how easy to introduce it in programming and after scale up, we will be able to come up with decisions on whether it will be accepted or not. Yes, we are going to take it further this conversation beyond dissemination because it has been accepted in a couple of countries in Africa.''












Comments