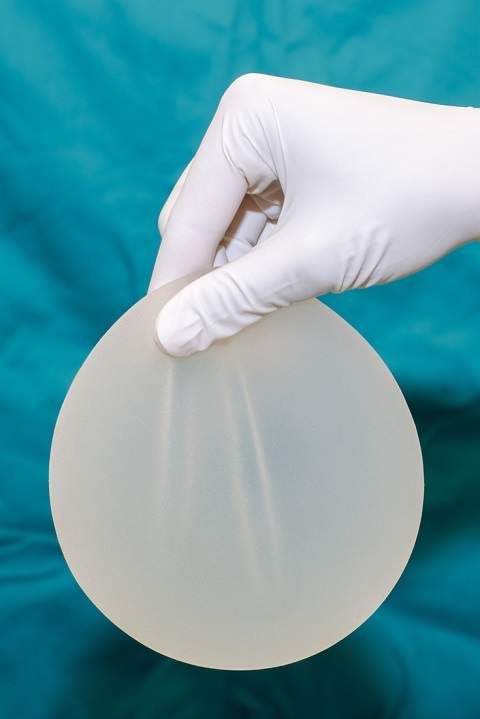
Silicone implants can increase a woman's risk of arthritis, stillbirth and even skin cancer, a new study suggests.
Researchers found that women had a 4.5-fold increased risk of having a stillbirth and a four times greater risk of developing melanoma after their procedures.
In comparison with saline implants, women with silicone implants were also two times more likely to have surgical complications, particularly scarring around the implant.
The team, from the University of Texas MD Anderson Cancer Center in Houston, says its study is the largest of breast implant outcomes to date and the findings are important to help women choose the implant they feel is right for them.
The most popular implants approved by the US Food and Drug Administration (FDA) are silicone implants and implants filled with a saline solution.
Silicone implants use shells filled with a plastic gel while saline implants use silicone shells filled with a sterile saline solution.
For breast reconstruction, the rebuilding of a breast, both implants are approved for women of all ages.
For breast augmentation, meant to increase the size or change the shape of a breast, saline implants are approved for women aged 18 or older and silicone implants are approved for women aged 22 or older.
Many women say silicone implants feel more like real breasts than saline implants do, but they pose a greater risk if they leak.
In the early 1990s, the FDA prohibited silicone implants after several health concerns were raised about their association to a risk of cancer, connective tissue disease and autoimmune diseases.
No research established a definitive link between silicone implants and these conditions.
After silicone implants from two manufacturers were approved in 2006, the FDA conducted many postapproval studies, but no researchers had analyzed the databasecom
For the new study, published in the journal Annals of Surgery, the team looked at nearly 100,000 patients enrolled in large postapproval studies between 2007 and 2010.
Approximately 80,000 had silicone implants and the rest received implants filled with a saline solution.
Additionally, 72 percent of the women had a breast augmentation, 15 percent had a revision augmentation, 10 percent had breast reconstruction, and three percent had revision reconstruction.
Researchers found the women who had silicone implants had a higher risk of several rare adverse outcomes.
This included rheumatoid arthritis; Sjogren's syndrome, an immune system disorder characterized by dry eyes and dry mouth; and sclerodermia, chronic hardening and tightening of the skin and connective tissues.
All these conditions came with a risk six to eight times higher in these women than in the general population.
Women with silicone implants were also at a 4.5-fold increased risk of having a stillbirth, but not a miscarriage.
Researchers also found that women with silicone implants had a risk of developing melanoma that was four times greater.
Silicone implants were also associated with a higher risk of surgical complications compared to saline solutions.
Around five percent of women had scarring around the implant, known as capsular contracture, compared with 2.8 percent of women with saline implants.
'We are reporting an analysis of the largest prospective study to date on silicone breast implant safety,' said Dr Mark Clemens, an associate professor in the department of plastic surgery at MD Anderson Cancer Center.
'We are sharing critical information on complication rates and rare associations with systemic harms. This data gives women important safety information about silicone breast implants to have real expectations and to help them choose what is right for them.'
The authors noted that although certain risks were more common in women with silicone implants, 'absolute rates of these adverse outcomes were low'.
'Women shouldn't panic but what it does tell us is that there's something going on and it's different than what women have been told for the last two decades,' Dr Diana Zuckerman, President of the National Center for Health Research, told DailyMail.com
'It's still cause for concern and you have to think that all those increased risks adds up. Not all women are getting all these diseases but it's some getting one and some getting another that could be statistically different.'
But Dr Stuart Linder, a plastic surgeon from Beverly Hills, California, is skeptical of the study.
'I use silicone implants and I have for over 20 year and I haven't seen anything that has hinted of this,' he told DailyMail.com.
'Unless the FDA states the risk is higher, then the products are deemed to be safe and usable in surgery.'














Comments